In 2013, Phillip (Phil) Landgraf faced several glaring problems in the financial performance of his company, BioPharma, Inc. The firm had experienced a steep decline in profits and high costs at its plants in Germany and Japan. Landgraf, the company’s president for worldwide operations, knew that demand for the company’s products was stable across the globe. As a result, the surplus capacity in his global production network looked like a luxury he could no longer afford.
Any improvement in financial performance was dependent on having the most efficient network in place, because revenues were unlikely to grow. Cutting costs was thus a top priority for the coming year. To help design a more cost-effective network, Landgraf assigned a task force to recommend a course of action.
1. Background
BioPharma, Inc. is a global manufacturer of bulk chemicals used in the pharmaceutical industry. The company holds patents on two chemicals that are called Highcal and Relax internally. These bulk chemicals are used by the company’s pharmaceutical division and are also sold to other drug manufacturers. There are distinctions in the precise chemical specifications to be met in different parts of the world. All plants, however, are currently set up to be able to produce both chemicals for any part of the world.
For 2013, sales of each product by region and the production and capacity at each plant are shown in Table 6-18. The plant capacity, measured in millions of kilograms of production, can be assigned to either chemical, as long as the plant is capable of producing both. BioPharma has forecast that its sales for the two chemicals are likely to be stable for all parts of the world, except for Asia without Japan, where sales are expected to grow by 10 percent annually for each of the next five years before stabilizing.

The Japanese plant is a technology leader within the BioPharma network in terms of its ability to handle regulatory and environmental issues. Some developments in the Japanese plant had been transferred to other plants in the network. The German plant is a leader in terms of its production ability. The plant has routinely had the highest yields within the global network. The Brazilian, Indian, and Mexican plants have somewhat outdated technology and are in need of an update.
2. Current Plant Costs at BioPharma
After considerable debate, the task force identified the cost structure at each plant in 2013 as shown in Table 6-19. Each plant incurs an annual fixed cost that is independent of the level of production in the plant. The fixed cost includes depreciation, utilities, and the salaries and fringe benefits of employees involved in general management, scheduling, expediting, accounting, maintenance, and so forth. Each plant that is capable of producing either Highcal or Relax also incurs a product-related fixed cost that is independent of the quantity of each chemical produced. The product- related fixed cost includes depreciation of equipment specific and other fixed costs that are specific to a chemical. If a plant maintains the capability to produce a particular chemical, it incurs the corresponding product-related fixed cost even if the chemical is not produced at the plant.

The variable production cost of each chemical consists of two components: raw materials and production costs. The variable production cost is incurred in proportion to the quantity of chemical produced and includes direct labor and scrap. The plants themselves can handle varying levels of production. In fact, they can also be idled for the year, in which case they incur only the fixed cost and none of the variable cost.
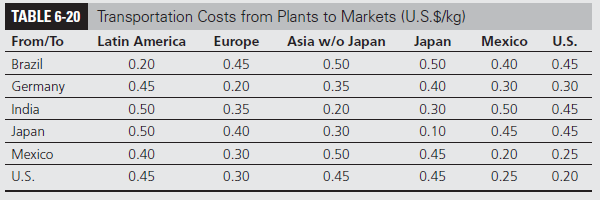
BioPharma transports the chemicals in specialized containers by sea and in specialized trucks on land. The transportation costs between plants and markets are as shown in Table 6-20. Historical exchange rates are shown in Table 6-21 and the regional import duties in Table 6-22. Given regional trade alliances, import duties in reality vary based on the origin of the chemical. For simplicity’s sake, however, the task force has assumed that the duties are driven only by the destination. Local production within each region is assumed to result in no import duty. Thus, production from Brazil, Germany, and India can be sent to Latin America, Europe, and the rest of Asia excluding Japan, respectively, without incurring any import duties. Duties apply only to the raw material, production, and transportation cost component and not to the fixed cost component. Thus, a product entering Latin America with a raw material, production, and transportation cost of $10 incurs import duties of $3.

3. Network Options Under Consideration
The task force is considering a variety of options for its analysis. One option is to keep the global network with its current structure and capabilities. Other options include shutting down some plants or limiting the capability of some plants to producing only one chemical. Closing down a plant eliminates all variable costs and saves 80 percent of the annual fixed costs (the remaining 20 percent accounts for costs that are incurred related to the plant shutdown). Similarly, if a plant is limited to producing only one chemical, the plant saves 80 percent of the fixed cost associated with the chemical that is no longer produced. The two options being seriously considered are shutting the Japanese plant and limiting the German plant to a single chemical.
Source: Chopra Sunil, Meindl Peter (2014), Supply Chain Management: Strategy, Planning, and Operation, Pearson; 6th edition.

Hey There. I found your blog using msn. This is a really well written article. I will be sure to bookmark it and come back to read more of your useful info. Thanks for the post. I’ll definitely return.